Discoveries
science in action
Schuller Group
Complex oxides: Vanadium Oxide
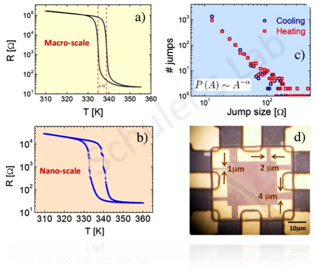
VO2 is a unique complex oxides due to its enhanced electron-electron interaction which has a major influence on the electric properties. VO2 exhibits a sharp electronic transition from an insulating state (room temperature) to a metallic state (high temperature) see Fig 1a. This transition is highly coupled with a structural transition above a temperature of 340 K.
The metal-insulator transition (MIT) in vanadium dioxide has been known for decades. Recently we were able to measure the transition at sub-micron scales, see fig. 1b,d. Our measurements indicate a self-organized critical behavior at the MIT which exhibites avalanches and percolation (Fig 1c).
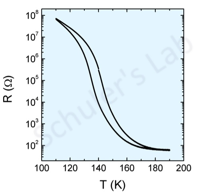
Other vanadium oxides also exhibit a MIT. V2O3 is a particular case where the MIT shows up around 150 K and an additional paramagnetic-antiferromagnetic transition occurs. First attempts at growth of a single-phase thin film show a 6 orders of magnitude change in resistance (fig 2 below).
References
- Amos Sharoni, Juan Gabriel Ramírez and Ivan K. Schuller, Phys. Rev. Lett. 101, 026404 (2008)
- J. -G. Ramírez, A. Sharoni, Y. Dubi, M. E. Gómez, and Ivan K. Schuller. Phys. Rev. B 79, 235110 (2009)